Background: Chronic anemia exerts profound impacts on the morbidity, mortality, and quality of life in individuals with sickle cell disease (SCD), but therapeutic options are limited. Erythropoietin-stimulating agents (ESAs) are the standard of care for managing anemia in chronic kidney disease (CKD). However, their utilization in SCD remains ad hoc, with limited literature and no guidelines supporting the use of ESAs in SCD. To address this knowledge gap, we present preliminary findings from a survey designed to evaluate provider practices and identify factors influencing the use of ESAs in SCD.
Methods: We conducted a cross-sectional web-based survey from March-July 2023 among providers taking care of patients with SCD in the United States (US) and internationally. Survey items were developed to assess provider demographic characteristics, practice environments, and provider practices around ESA use in SCD. The data was collected and managed using REDCap electronic data capture tools hosted at the University of Pittsburgh. Descriptive statistics were used to report the data.
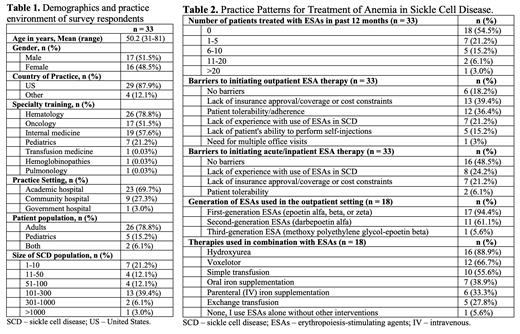
Results: The survey has been completed by 33 providers as of a cut date of July 23, 2023. Most providers were hematologists who practiced in an academic setting (23/33, 69.7%), with an equal gender distribution (Table 1). The number of patients with SCD seen annually varied, with 101-300 patients being the most common cohort size (13/33, 39.4%).
In the 12 months prior to survey completion, 15/33 (45.5%) providers treated at least 1 patient with SCD with ESAs, usually outpatient (Table 2). Commonly reported barriers to outpatient ESA therapy included lack of insurance approval/coverage or cost concerns, lack of provider experience, and patient tolerability/adherence or inability to perform self-injections. Fewer respondents reported no barriers to prescribing ESAs in the outpatient setting, compared to in the acute/inpatient setting (18.2% vs. 48.5%). The most commonly reported indications for starting ESAs in the outpatient setting were chronic anemia from end-stage renal disease or CKD, reduction of blood transfusion requirement, and chronic anemia in patients refractory to approved therapies. Less commonly reported indications include severe or symptomatic anemia alone and chronic anemia in the setting of cardiopulmonary disease. Respondents reported several contraindications to starting ESAs, including a history of venous thromboembolism (VTE), malignancy, stroke, coronary artery disease, hypertension, and frequent vaso-occlusive crises. Respondents also expressed concerns about the cost/insurance coverage, efficacy, safety, patient burden, and appropriate dosing of ESAs in patients with SCD.
Only 18/33 (54.5%) respondents reported ever treating outpatients with SCD with ESAs; nearly all of them used 1 st generation ESAs, and a majority used 2 nd generation ESAs. For 1 st generation ESAs, the most common starting dose was 3500-7000 units (U) (50-100 U/kg; 22.2%) or 7001-14000 U (101-200 U/kg; 22.2 %). Once weekly was the most common dosing interval (50%), followed by three times a week (TIW; 27.8%) and twice a week (16.7%). The maximum dose used was 56001-70000 U (801-1000 U/kg) TIW. For 2 nd generation ESAs, the most common starting dose was 141-210 mcg (2.1-3.0 mcg/kg) (22.2%), and administration often occurred once every 2 weeks (44.4%) or weekly (27.8%). The maximum dose used was 211-315 mcg (3.1-4.5 mcg/kg) weekly. Outpatient ESAs were most often initiated for hemoglobin (Hb) < 6 g/dL (8/18, 44.4%), and Hb > 9 g/dL was the most common treatment target (6/18, 33.3%). ESAs were often combined with hydroxyurea, voxelotor, and simple transfusions (Table 2).
Conclusion: These preliminary survey results show considerable variability in how ESAs are currently being used in people with SCD. These variations in clinical practice highlight both significant ambiguity about what works in this setting and the need for further research into the optimal use of ESAs for the management of chronic anemia in SCD (ongoing clinical trial: ACHiEvE-SCD, NCT05451940). Future directions include evaluating differences in practice patterns among adult vs. pediatric and US-based vs. non-US-based providers.
Disclosures
Novelli:Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees. Decastro:Novartis: Other: Advisory board; Global Blood Therapeutics: Other: Advisory board; GlycoMimetics: Other: Advisory board; Forma Therapeutics: Other: Advisory board. Xu:Agios Pharmaceuticals, Inc.: Other: US national principal investigator for the Phase 1 clinical trial pf AG-946 in patients with sickle cell disease.; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal